Scientific background
Ionic liquids
Ionic liquids are organic salts with a melting point below 100 °C. They are composed of a cation (Figure 1 left) and an anion (Figure 1 right). Their low melting point is due to a disturbance of the long range order of the crystal lattice due to steric hindrance or charge delocalization which causes a reduction of the lattice energy. Ionic liquids are highly variable in their chemical composition. Therefore, many material properties can be tailored for an application through the choice of cation and anion species.
 Figure 1. Typical cations (left) and anions (right) in ionic liquids.
Figure 1. Typical cations (left) and anions (right) in ionic liquids.
By variation of the side chains and introduction of functional groups on the cation multifunctional ionic liquids can be synthesized. The synthesis of the cations (Figure 2) is usually done by quaternization of amines or phosphines with alkylating agents or acids.
Figure 2. Typical synthesis of an ionic liquid from an amine.
Typical basis of the cations (Figure 1 left) are ammonium, imidazolium, guanidinium, piperidinium and pyridinium ions. Halides, tetrafluoroborate, tetrachloroaluminates, acetates, trifluoroacetate, triflates, tosylates and hexafluorophosphates serve as base of the anions (Figure 1 right). By the addition of paramagnetic salts such as iron(III) chloride magnetic ionic liquids can be prepared. Due to their chemical structure, ionic liquids can be used as a replacement for organic solvents, as phase transfer catalysts in Suzuki reactions for catalysis or as a solvent for recycling in cellulose of composite packaging. The purity of the ionic liquid is essential for their chemical and physical properties. In turn, the purity plays an important role in the use of an ionic liquid as a non-volatile solvent. Because of the non-volatile nature of ionic liquids purification by distillation is very difficult, the synthesis of highly pure, binary ionic liquids is desirable by optimizing the reaction conditions.[1;2]
State of research
Synthesis of ionic liquids
The synthesis of ionic liquids is described in many books and publications with homogeneous or heterogeneous catalysts and in many cases, at high temperatures, high pressure and over a long period of time up to several days. Due to the very low vapor pressure of ionic liquids, it is not possible to purify them by distillation. Therefore, it is reasonable to synthesise ionic liquids with equimolar amounts of starting materials in micro structured reactors in continuous flow. In addition to difficulties in processing, the direct synthesis of ionic liquids with unusual anions is not possible. Therefore, multi-step syntheses are necessary, in which the desired anion is exchanged with the halogen anion of an ionic liquid. For a few years, the alkylation of N methylimidazole (MIM) with dimethyl carbonate (DMC) is investigated. Dimethyl carbonate is known as a clean methylation agent which can be used as safe alternative to conventional reagents such as methyl halides, dimethyl sulfate and phosgene. With the system shown in Figure 4, which is operated continuously, it is possible to synthesize the zwitterion 1,3-dimethyl-1H-imidazol-3-ium-2-carboxylate ([MMIM][CO2]) by alkylation of MIM with DMC. Similarly, the synthesis of ionic liquids based on N methylpiperidine and N methylpyrrolidine is possible. These ionic liquids represent versatile precursors for the synthesis of halogen free ionic liquids.
 Figure 3. Flow chart carboxylate system (B = vessel, V = valve).
Figure 3. Flow chart carboxylate system (B = vessel, V = valve).
Applications of ionic liquids
a) As a solvent for cellulose
Ionic liquids, such as 1 ethyl 3 methylimidazolium acetate ([EMIM][OAc]) and 1 butyl 3 methylimidazolium chloride ([BMIM][Cl]), are described in the literature as good solvents for cellulose.[3;4] At this state of research the solubility of pure cellulose, filter paper, newspapers, composite packaging and paper containing waste in thirteen ionic liquids was investigated. The focus was placed on ionic liquids based on imidazolium. Figure 5 (l to r) shows the pure, undissolved cellulose and the completely dissolved cellulose in [MMIM][OAc]. After precipitation with water or ethanol the cellulose can be separated by centrifugation and dried after removal of the ionic liquid. The IL can be reused after purification by distillation.
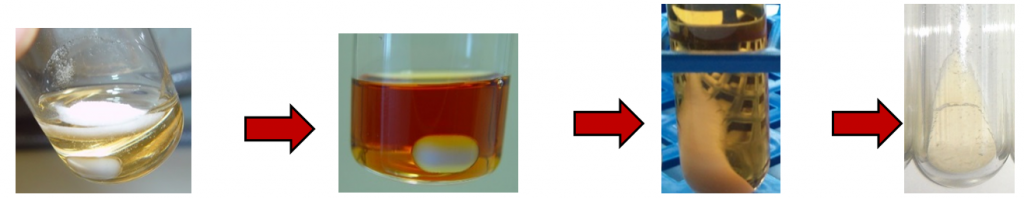 Figure 4. Overview of solution and precipitation of cellulose in [MMIM][OAc]
Figure 4. Overview of solution and precipitation of cellulose in [MMIM][OAc]
b) As a phase-transfer catalyst in Suzuki cross-coupling reactions
The Suzuki cross-coupling reaction is widely used to generate carbon-carbon bonds from boronic acids and halides. It is successfully applied in homogeneous and heterogeneous synthesis of e.g. biaryls.[5] The formation of two phases during the reaction requires intensive mixing in batch. After transfer to a flow system, a continuous mixing along the flow axis is required to stabilize the optimized interfacial area (Figure 8). Furthermore, pressurized systems are required to enable high temperatures above the boiling point in the capillaries or tubes. For these purposes a system was built (Figure 5) which allows working conditions up to 120 bar and 180 °C. To enlarge the interfacial surface and consequently increase the mass transfer by abiding the dispersion, a newly developed residence time-mixing-element filled with SIGRATHERM® Soft Felt was used (Figure 6). Using this micro system a nearly quantitative coupling of bromoarenes with a very low concentration of palladium catalyst was achieved in a few minutes. Using an ionic liquid as phase-transfer catalyst under batch conditions causes the stabilization of the aryl boronats and the generation of an educt-product-catalyst three-phase system and leads to products in high purity as it is known in the literature.[6] With the addition of [C18MIM][Br] the coupling was done in 100 seconds and allows repetitive catalytic runs because the ionic liquid contains the activated catalyst.
 Figure 6. Left: REM picture of SIGRATHERM® Soft Felt; right: Model of the moistened SIGRATHERM® Soft Felt
Figure 6. Left: REM picture of SIGRATHERM® Soft Felt; right: Model of the moistened SIGRATHERM® Soft Felt
Acknowledgment
We acknowledge financial support by the AiF Projekt GmbH grant funded by the Bundesministerium für Wirtschaft und Energie.
Reference List
[1] P. Wasserscheid, T. Welton, Ionic Liquids in Synthesis, 2003.[2] P. Wasserscheid, R. van Hal, A. Bosmann, J. Esser, A. Jess, Ionic Liquids as Green Solvents: Progress and Prospects, 2003, p. 57.
[3] R. P. Swatloski, S. K. Spear, J. D. Holbrey, R. D. Rogers, J.Am.Chem.Soc. 2002, 124 4974-4975.
[4] A. Pinkert, K. N. Marsh, S. Pang, M. P. Staiger, Chem.Rev. 2009, 109 6712-6728.
[5] K. R. Seddon, J.Chem.Technol.Biotechnol. 1997, 68 351.
[6] C. J. Mathews, P. J. Smith, T. Welton, Chem.Commun. 2000, 1249-1250.

